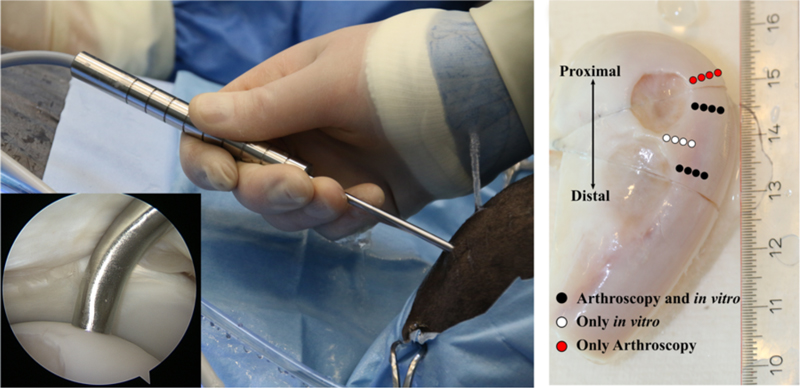
As part of his PhD, Jaakko Sarin’s of UEF developed an arthroscopic near infrared spectroscopic probe for evaluation of articular cartilage and subchondral bone structure and composition. The availability of comprehensive information on the health of joint tissues could substantially enhance the treatment outcome of arthroscopic intervention.
Osteoarthritis (OA) is a disabling disease characterized by joint pain and restricted mobility. The disease generally progresses slowly, even over decades, and is most prevalent in articulating joints, such as the knee.
Although currently no cure exists for OA, early detection of cartilage lesions could enable halting disease progression. Conventionally, joint health is diagnosed based on patients’ symptoms, joint mobility and, if required, with x-ray and magnetic resonance imaging. Based on these examinations, joint repair surgery may be performed during arthroscopy.
The decision on the optimal treatment option is made during the surgery, in which the joint health is evaluated visually and by palpating the cartilage surface with a metallic hook. These techniques are subjective and dependent on surgeons’ experience and can, therefore, influence the treatment outcome.

Previously, the near infrared spectroscopy technique has been used, for example, in the evaluation of grain quality, but its clinical applications are still rare. However, clinical application of the technique is now possible, thanks to better availability of computational power along with state-of-the-art mathematical modelling methods, such as neural networks.
With these methods, the relationship between the absorption of near infrared light and tissue properties can be determined. This enables reliable determination of articular cartilage stiffness and subchondral bone mineral density — changes in these tissue properties are prognostic indicators of OA.