For a purposeful research and development, apart from a corresponding scientific excellence, the knowledge of observing norms and regulations is essential for the success of a consequential product development. To promote an early interaction of the various disciplines, the EU organized the workshop “Performance Assessment and Standardization in Biophotonics” on the 26th of October of 2018 in Auderghem, Belgium.
A selection of outstanding speakers spoke about their previous experience in the field of calibration, instrument performance, publication and standardization (e.g. in the field of blood oxygen measurement, mammography). Different strategies were described, which can be transferred for the development of biophotonic products for medical applications with the goal of avoiding, or at least minimizing, the often cited valley of death in product development.
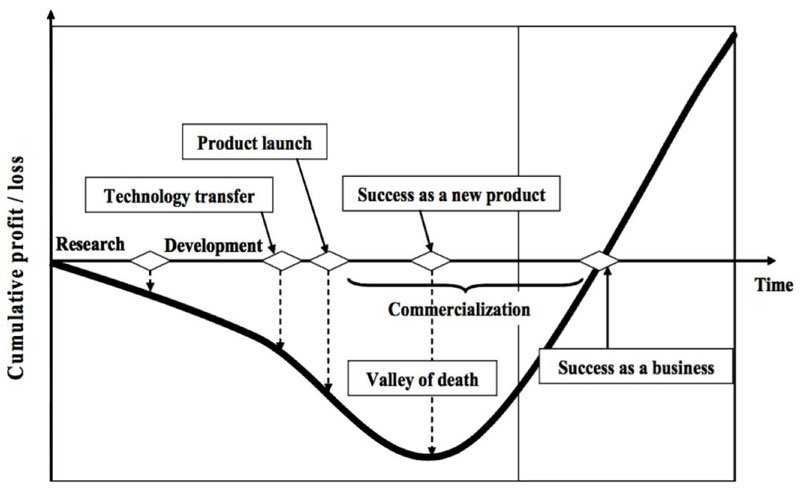
Valley of Death (Osawa & Miyazaki, 2006).
OptoPrecision GmbH participated in this workshop as a representative of the MIRACLE consortium and collected very helpful information not only to OptoPrecision GmbH, but to the whole consortium. Furthermore, some personal contacts were established with the experts present, which could be useful in the exploitation of the MIRACLE project results. In particular, the issue of reproducible instrument calibration with corresponding phantoms (which so far does not exist with regard to the MIRACLE measurement task), as well as conformity with national and international standards were identified as very important points, and should be pursued with a high priority.